Applying proteomics to Parkinson's
(Medical Xpress)—Scientists studying two genes that are mutated in an early-onset form of Parkinson's disease have deciphered how normal versions of these genes collaborate to help rid cells of damaged mitochondria. Mitochondria are the cell's primary energy source, and maintaining their health is critical for cellular function. Mitochondrial dysfunction may underlie multiple neurodegenerative diseases, including Parkinson's.
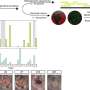
In their analysis published in Molecular Cell, Harvard Medical School researchers used powerful quantitative mass spectrometry and live-cell imaging approaches to elucidate a multistep mechanism by which the two proteins mutated in Parkinson's disease—PINK1 and PARKIN—mark mitochondria as damaged by attaching chains of a small protein called ubiquitin. This work paves the way for a deeper understanding of what molecular steps are defective when these proteins are mutated in patients with Parkinson's disease.
"The PINK1-PARKIN pathway has been studied for many years, yet its mechanisms weren't clearly defined," said Wade Harper, Bert and Natalie Vallee Professor of Molecular Pathology in the Department of Cell Biology at HMS and senior author of the paper. "Combining imaging and advanced mass spectrometry approaches has allowed us for the first time to determine with molecular precision the biochemical output of the PINK1-PARKIN pathway in living cells."
One hypothesis about the origin of Parkinson's disease suggests that neurons place high energy demands on their mitochondria. When mitochondria become damaged and their energy production falls, they must be cleared away; if not, cell death results when the damaged mitochondria create harmful chemicals called reactive oxygen species.
People who have certain early-onset mutations in PINK1 or PARKIN genes may live normal lives until they enter their 30s when movement disorders begin to appear, reflecting the loss of neurons that make the neurotransmitter dopamine. These neurons seem to be the cells that are the most sensitive to an inability to remove damaged mitochondria.
Only in the last few years have scientists understood that the enzymes PARKIN and PINK1 work together to remove damaged mitochondria. The PINK1 kinase, an enzyme that transfers phosphate to other proteins, is activated specifically on damaged mitochondria where it then functions to promote accumulation of PARKIN on the mitochondrial surface. Once there, PARKIN—a ubiquitin ligase— marks numerous proteins on the surface of the mitochondria with chains of ubiquitin, which in turn target the damaged mitochondria for removal from the cell.
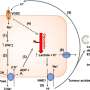
In their new work, Harper's team identifies a multistep "feed-forward" mechanism that involves intertwined ubiquitylation and phosphorylation in a sequence of reactions that successively build on one another. To the authors' knowledge, this is the first report of a feed-forward mechanism of this type.
The team, led by postdoctoral fellow Alban Ordureau, found that PINK1 actually has two functions in a multistep pathway. First, PINK1 phosphorylates PARKIN, greatly stimulating its ability to attach ubiquitin to mitochondrial substrates. Second, PINK1 phosphorylates ubiquitin chains that PARKIN has just built. Unexpectedly, these phosphorylated ubiquitin chains then bind tightly to activated PARKIN, thereby facilitating its retention on the mitochondrial surface and furthering ubiquitin chain assembly through a feed-forward mechanism. Eventually these chains become so dense that the damaged mitochondria are marked for degradation.
"Our finding that PARKIN binds phosphorylated-ubiquitin chains as its mechanism of retention on damaged mitochondria was completely unexpected," Harper said. "Ubiquitin has been studied for almost 40 years, but only recently has regulation of ubiquitin by phosphorylation emerged as a major focus for the field."
Methods employed in this study have their origins in prior work of Steven Gygi, HMS professor of cell biology and a co-author of the paper, who developed ways to quantify ubiquitin chains more than a decade ago. Harper says there is "enormous potential in the application of these approaches to understand how defects in the ubiquitin system lead to disease."
The team also included Brenda Schulman, a Howard Hughes Medical Institute investigator, the co-director of the Cancer Genetics, Biochemistry and Cell Biology Program at St. Jude Children's Research Hospital and a leading expert on ubiquitin biochemistry.
"This is a very intricate pathway," Ordureau said. "We were surprised by our findings at every step."
More information: "Quantitative Proteomics Reveal a Feedforward Mechanism for Mitochondrial PARKIN Translocation and Ubiquitin Chain Synthesis." Alban Ordureau, et al. Molecular Cell. DOI: dx.doi.org/10.1016/j.molcel.2014.09.007